September 5, 2024
Making Use Of A Phenotype-guided Method For The Treatment Of Obesity

Tesofensine Understanding And Referrals The loss of leptin causes extreme metabolic disruptions, which include extreme hyperphagia, lipodystrophy and hypothalamic amenorrhoea136,213. Numerous clinical research studies validated the performance of rDNA-derived human leptin for the treatment of hypothalamic amenorrhoea214,215 and leptin supplements in ob/ob computer mice is sufficient to recover fertility216. Nonetheless, although leptin supplements works in people with congenital leptin deficiency, the hormone shows little capability to reduced body weight under problems of typical, polygenetic, obesity115,116,137,138. Likewise, regardless of not being correlative to lower efficacy or safety, the growth of antibodies against metreleptin makes up an obstacle for its scientific use219.
21 Representatives That Have Actually Gotten To Phase 3 Clinical Trials
Another popular failing of an AOM was sibutramine-- a norepinephrine and serotonin reuptake inhibitor that lowers appetite and promotes thermogenesis. Sibutramine was authorized by the FDA in 1997 yet was taken out because of raising the danger of cardio occasions in a high-risk populace for which sibutramine's usage was initially not intended154. To attend to the possibility for damaging cardio occasions, the precursor test was started to establish lasting cardio end results in a high-risk population. Amazingly, the occurrence of non-fatal heart attack and non-fatal stroke was dramatically greater in patients treated with sibutramine156,331, although various other researches recommended that sibutramine is fairly risk-free in individuals without greater threat for a cardio event153,154,332. Although cardiovascular safety and security problems ended further use https://s5d4f86s465.s3.us-east.cloud-object-storage.appdomain.cloud/clinical-trials/product-sustainability/treatment-of-obtained-hypothalamic-obesity-currently-and-the.html sibutramine, fenfluramine and phenylpropanolamine, a battle with unfavorable mental effects emerged in other places. One noticeable example right here is rimonabant, an endocannabinoid 1 receptor (CB1) villain shown to decrease hunger, boost thermogenesis and decrease lipogenesis preclinically and in countless human trials333.What is tesofensine made use of for?
Randomized Regulated Test Of Tesomet For Weight-loss In Hypothalamic Weight Problems
- There has actually been considerable passion in this investigational medicine for weight reduction as an accessory to energy limitation.
- An equivalent outcome resulted in the use of anti-ghrelin Spiegelmers developed at NOXXON Pharma that only moderately boosted metabolic rate in preclinical studies, with no impact on food intake after 8 days of treatment246.
- This activity of buproprion was accompanied by a rise in dopamine degrees and in the phosphorylated type of the dopamine and cyclic-AMP associated phosphoprotein 32 kDaltons (pDARPP-32) in the nucleus accumbens core (Randall et al., 2014).
- As a result of the function of DA in the incentive brain system, it can trigger manic episodes in bipolar illness.
- Although under task of the benefit pathway can lead to frustration and low mood, excessive excitement can be addicting and stimulants are recognized as medicines of abuse.
Duty Of The Striatal Dopamine, Gaba And Opioid Systems In Moderating Feeding And Fat Intake
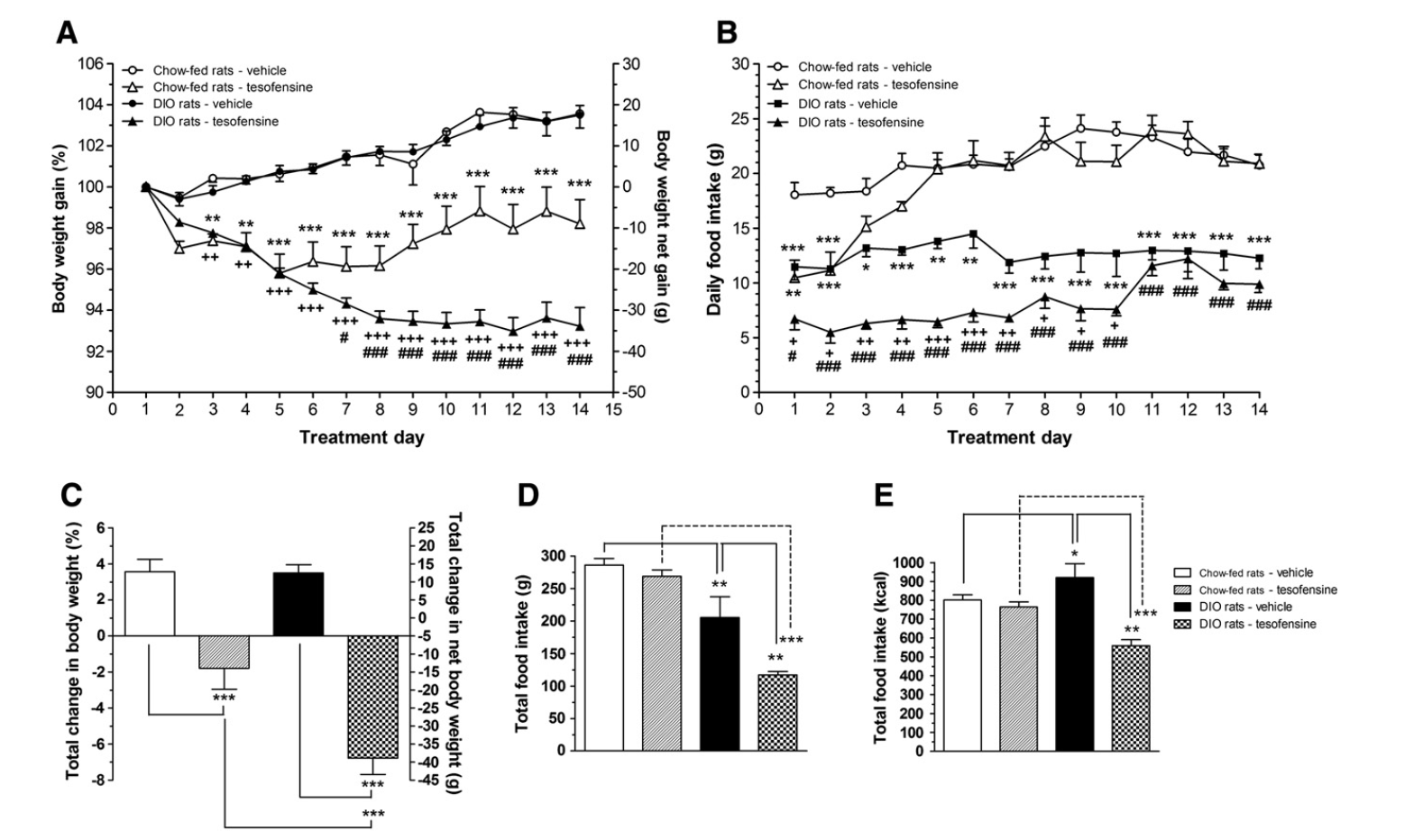
In general, 314 clients were screened; 60 people were left out mainly since their everyday off time did not drop between 2.0 and 6.0 hours or because they had clinically significant electrocardiographic irregularities. 3 of these individuals did not have an efficacy evaluation; as a result, the full-analysis collection comprised 251 clients. Seventy of 254 patients (27.6%) discontinued therapy too soon, mainly due to adverse events (53 individuals [20.9%]. The percents of patients who too soon took out because of damaging occasions were 22.4%, 11.5%, 25.0%, and 27.1% in the groups receiving tesofensine, 0.125, 0.25, 0.5, and 1 mg, respectively, compared with 18.4% in the sugar pill group. Client demographics, standard illness characteristics, and concomitant PD therapy are given up Table 1. The FDA advises that if a weight decrease of less than 3% is accomplished after 12 weeks of use, the medicine needs to be either discontinued or the dosage raised. If the patient does not attain a 5% weight decrease 12 weeks after a dosage rise, it is advised that this medicine must be progressively terminated. In the second endpoint analysis of all professional trials, the phentermine/topiramate CR group revealed considerable renovations in cardiometabolic threat aspects, including waist area, glycemic control, and lipid profile [37,38] Potential anti-obesity drugs in stage 3 professional tests are presented in Table 2 and reviewed below.Social Links